
Enables
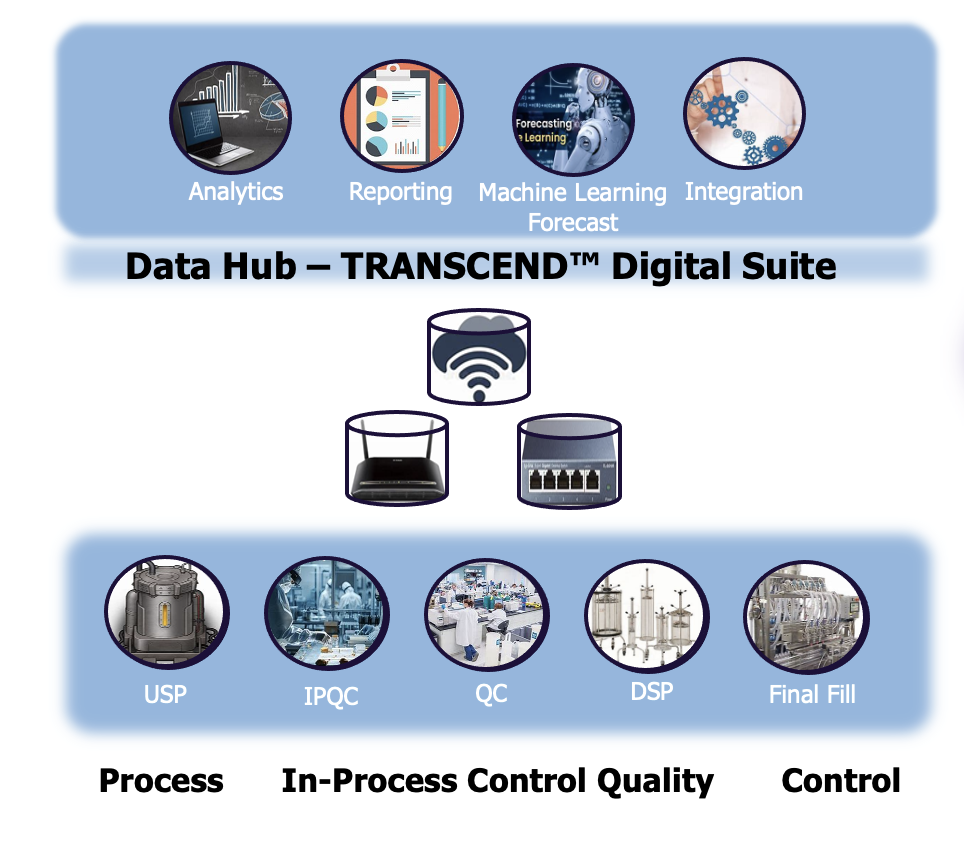
Specifically conceived by quality and process experts for process digitalization, TRANSCEND Software Suite is comprised of stand-alone, but seamlessly compatible software products linking all the data sources.
The intuitive interfaces and easy access enable a broad user base to share data and collaborate with global teams across geographies and organizational silos to connect, monitor, and analyze equipment, processes, results, and data in near real-time.

Multi unit operations involving splits and combinations
Uncertainty due to biological processes (inherent variability)
Changing correlation structures between unit operation
Designed and proven to meet automation and pharmaceutical Industrial Standards

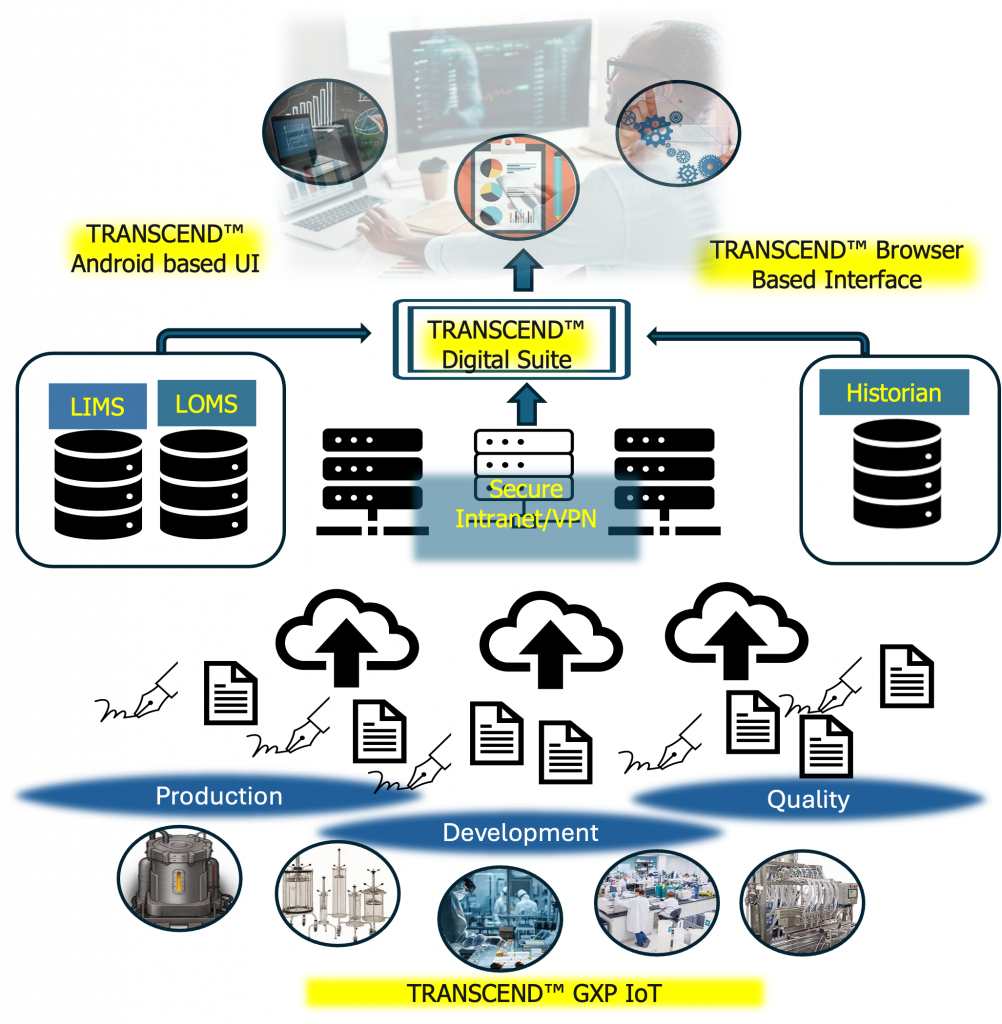
Through data aggregation, management, visualizations, and analysis, TRANSCEND™ Digital Suite makes all critical bioprocess data readily available.
TRANSCEND™ Digital Suite drives a sustainable operation through full digitalized reporting capability with ready to use and customized template
Enables true Paperless operations to meet ESG requirements
Reporting

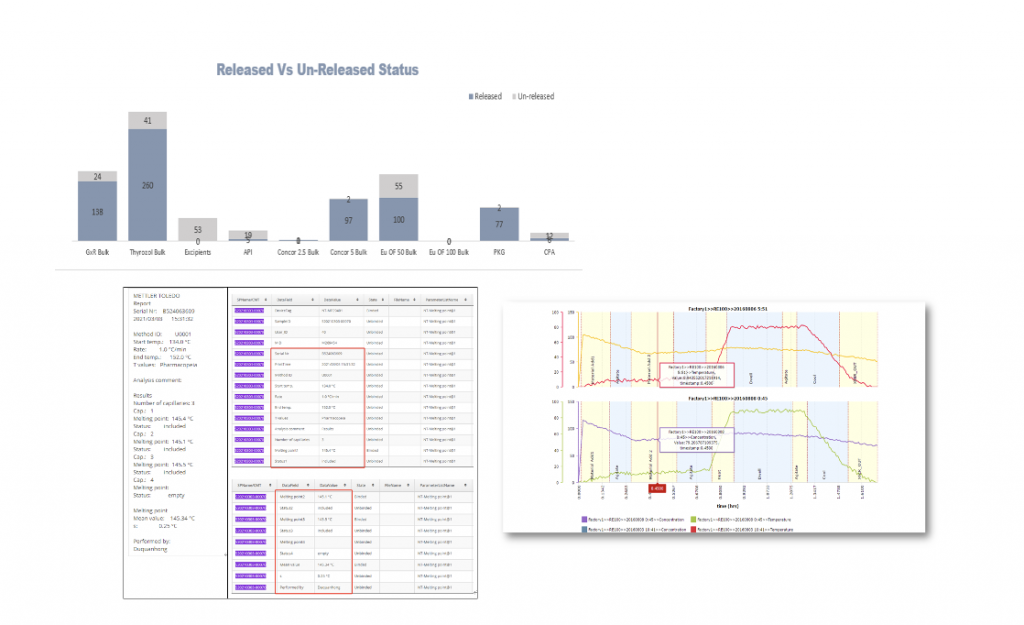
Process Monitoring & Visualization
Continued Process Verification (CPV) and Analytics